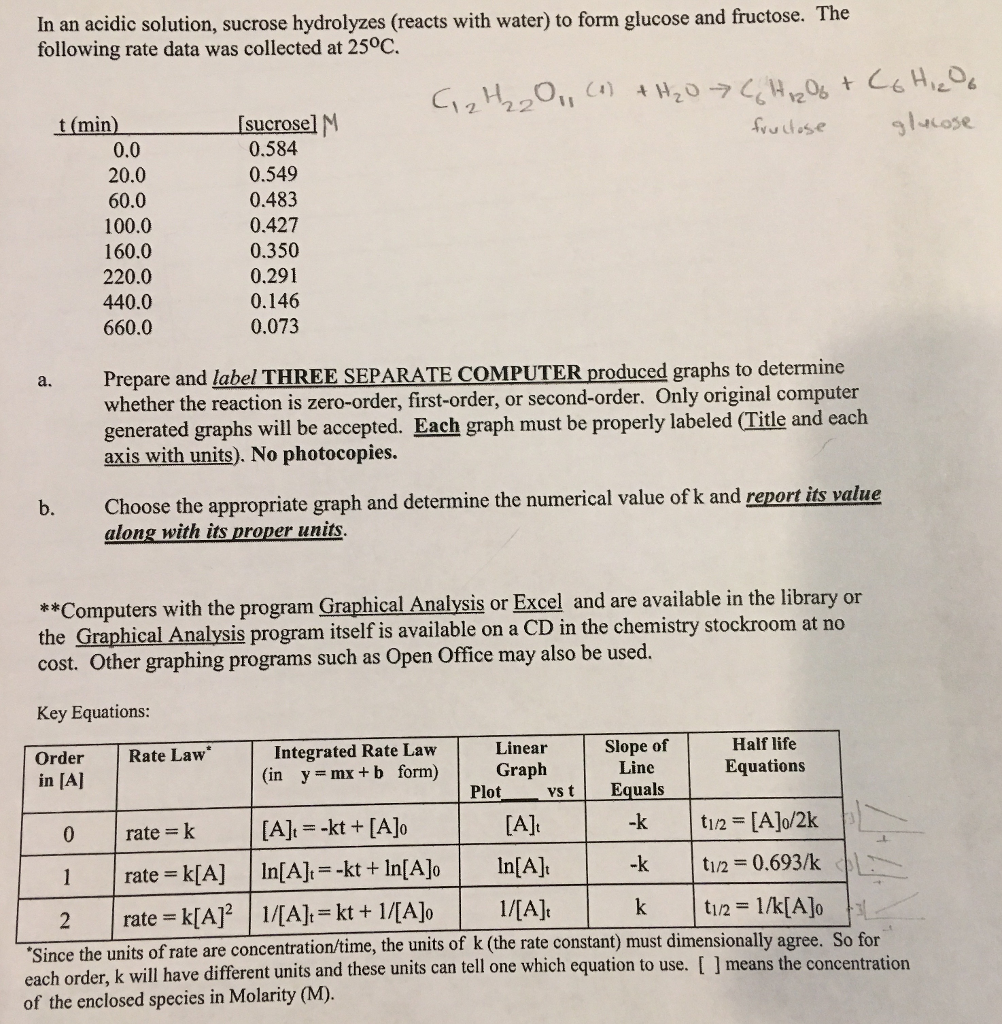
K Units For First Order Reaction
K Units For First Order Reaction. A first-order reaction is a reaction that proceeds at a rate that depends linearly on only one reactant concentration. We want to make sure all the units cross out so we get the units we need to Depending on, get to actually look at your data to see what time is, in this particular problem.

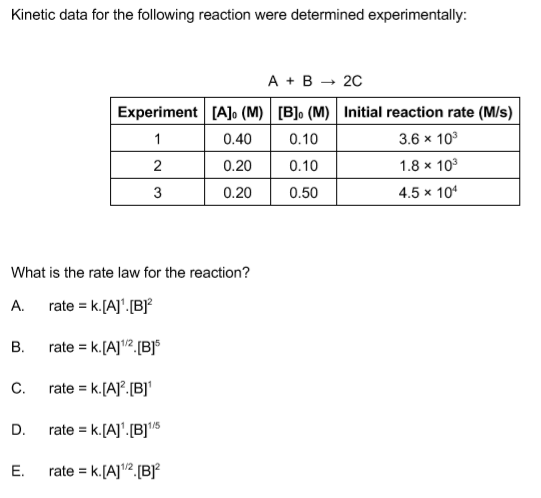
Rate laws may exhibit fractional orders for some reactants, and negative reaction orders are sometimes observed when an increase in the concentration of one reactant causes a decrease in reaction rate.
This reaction is first order with respect to A and zero order with respect to B, because the concentration of B doesn't affect the rate of the. > Chapter. > Rates Of Reactions And Chemical Kinetics. > For a first order reaction, un.
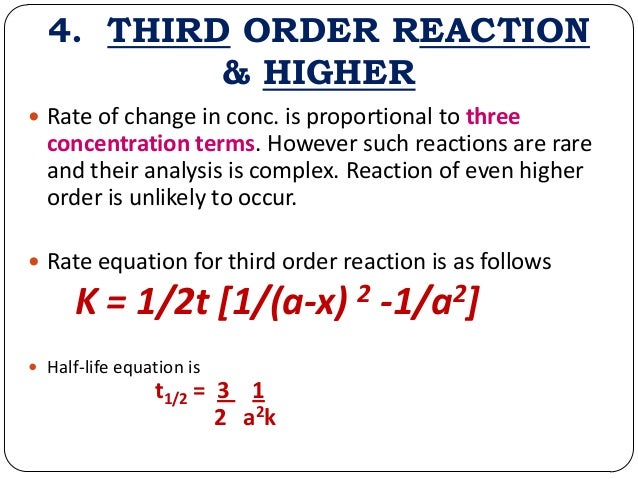
The rate constant of a first order reaction has only time unit. For a first order reaction, this is going to be the units for. In some of our examples, the reaction orders in the rate law happen to be the same as the coefficients in the chemical equation for the reaction.

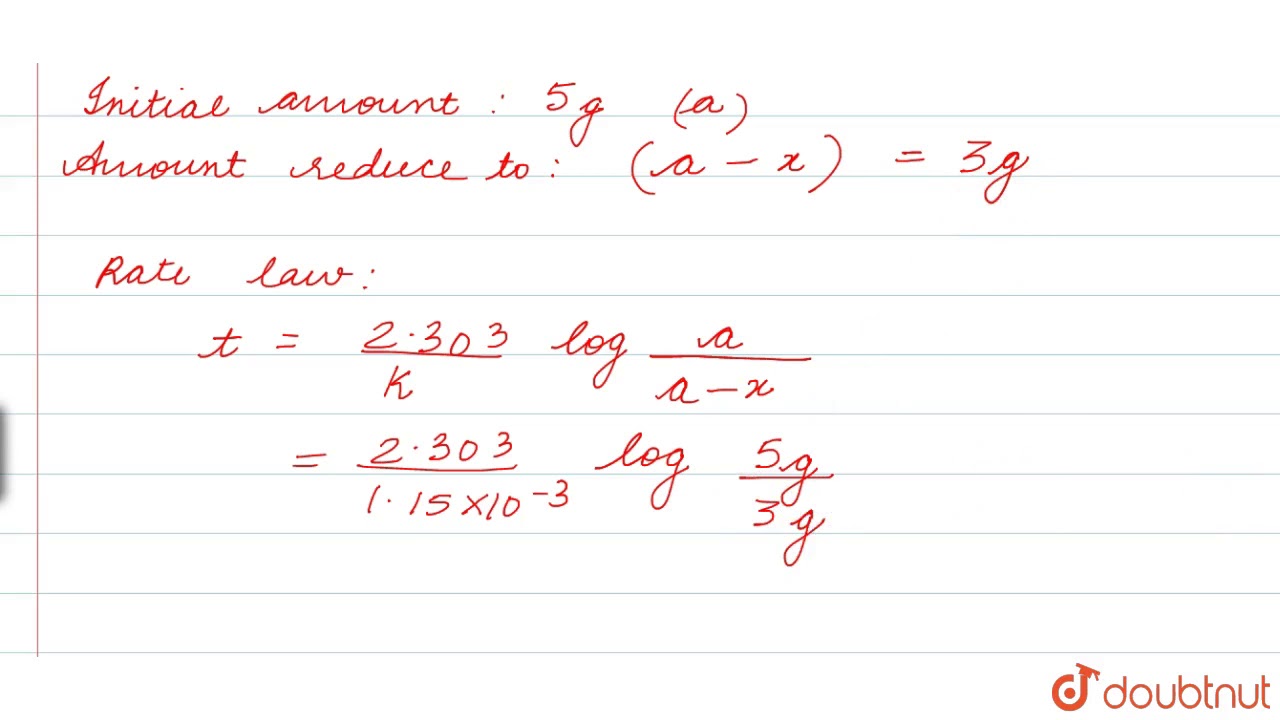

0 Response to "K Units For First Order Reaction"
Posting Komentar